Keep your kids’ brains busy and inspire innovation with simple STEAM projects you can do with the materials you have at home!
Just Added
Water Volcano in a Bottle
Have you ever tried to blow up a balloon in a bottle? When you try to blow the balloon up inside an “empty” bottle, you can’t because the bottle is actually already filled with air! Our lungs don’t exert enough pressure to force the air out of the bottle. These experiment will show you a tiny trick to play with pressure and inflate the balloon while creating a water volcano!
Learn more: Water Volcano in a Bottle
Cornstarch Slime

If you’ve ever wondered why it might be hard to get out of quicksand, it’s because it can act like both a liquid and a solid. Cornstarch slime is a fun substance that does this too! When a substance like this has properties of both liquids and solids, they are called non-Newtonian fluids, which you’ll learn more about in this experiment. You’ll also explore what interactions make it go from one state to another. When you slowly press your fingers into cornstarch slime —stored in a plastic bag —it’ll feel like a liquid. But when you quickly press your fingers into it, it becomes as hard as a rock!
Learn more: Cornstarch Slime
Glowing Oobleck

Like the Cornstarch Slime above, Oobleck is a type of mixture called a non-Newtonian fluid. If you push hard on it (no need to use hammers for this part), it hardens up and pushes back against you. If you push lightly on the oobleck, though, it stays liquidy and your hand will easily go into it. In this experiment, you’ll add fluorescent liquids that energize and make a delightful glow-in-the-dark activity!
Learn more: Glowing Oobleck
Make a Tornado in a Jar
Use mason jars and plastic water bottles to make your own mini tornadoes. Spin the bottles and watch your tornadoes twirl!
Learn more: Make a Tornado in a Jar
Get Creative with Food Coloring
Milk Swirl Experiment

You only need three ingredients to create an explosion of colors that will keep your kiddos entertained! This experiment will teach you about the special property of the soap molecules. When the soap touches the milk, it starts grabbing as many fat and protein molecules as it can. The attraction between the soap and the fat causes the molecules to move quickly, creating the colorful explosions. Follow the instructions or watch our video to see how to do it yourself.
Learn more: Milk Swirl Experiment
Ice Experiments Lab Book (printable)

Investigate the scientific process with ice! Our Kiwi Crate lab book has six awesome and easy experiments that will keep your kids busy while you get work done. The best part is you can download or print out all the activities!
Learn more: Ice Lab
Fizzing Colors

Who says chemistry has to be complicated? You only need three ingredients for this experiment: baking soda, food coloring, and vinegar. When you combine the ingredients, a chemical reaction occurs and results in a bubbling eruption of fizzing color!
Learn more: Fizzing Colors
Frozen Color Mixing

Experiment with color creation! Primary colors (red, blue, and yellow) are the source of all other colors. Secondary colors (green, purple, and orange) are created when two primary colors mix. If you freeze up colored ice cubes, you can experiment with color mixing as they melt.
Learn more: Frozen Color Mixing
Color Mixing with Slime

When we think of slime, we think of a super-fun gooey experience. But with plastic bags, we can create an awesome rainbow project that lets us enjoy slime and learn at the same time! In this experiment, we make slime in the three primary colors (red, yellow, and blue) and layer them up to produce secondary colors (orange, purple, and green).
Learn more: Color Mixing with Slime
Edible Paint

Make edible homemade finger paint using simple ingredients from the pantry. Practice color matching with paint and pieces of cut fruit to make a fun mixed-media art project.
Learn more: Edible Paint
Cook with Chemistry & Experiment with Food
Gummy Bear Science Project (printable version)

This experiment explores osmosis! That’s a chemistry term for the motion of water through a barrier (like a gummy bear). Watch as gummy bears grow and shrink in different liquids. You’ll start to see results in just a few hours, and you’ll definitely see big changes in size in just a day.
Learn more:Gummy Bear Science Project
Printable version: Growing Gummies
Invisible Ink with Lemon Juice

Heating up lemon juice causes some of its sugars to react with oxygen in the air (oxidation), which turns these sugars brown. Acids in the lemon juice can also react with fibers in the paper to make more sugars, which again turn brown in the heat. Your invisible ink might seem like magic, but really, it’s all just chemistry.
Learn more: Invisible Ink with Lemon Juice
Hopping Grains

In this experiment, you’ll observe how carbon dioxide bubbles stick to the grains in the glass, lifting them up to the surface of the water. But when the bubbles reach the surface, they pop. Without the bubbles to hold them up any more, the grains sink back down to the bottom. Then the whole process repeats, giving you hopping grains in a cup!
Learn more: Hopping Grains
Apple Science

Have you ever noticed that if you slice an apple in the morning, it turns brown by lunch? This is actually a chemical reaction at work! In this experiment, you’ll learn more about how the oxygen in the air around us causes this reaction (also known as oxidation). Test different liquids to see if you can figure out a way to keep apples fresh from morning to noon.
Learn more: Apple Science
Square Egg

When you hard boil an egg, molecular chemistry is at work. Eggs are made up mostly of two kinds of molecules: protein and water. The proteins in a raw egg are like twisted strings, floating in a watery soup. When you heat up an egg, the proteins break their bonds and unfold before making new, stronger chemical bonds between each other. When they’re still hot, though, the bonds between the proteins are moldable, kind of like clay. Try it out in this project and mold an abstract egg!
Learn more: Make a Square Egg
Egg in Vinegar Experiment

When you submerge a raw egg in the vinegar, you’ll see bubbles forming on the surface. You’re seeing a reaction between a compound in the eggshell (calcium carbonate) and an acid in the vinegar (acetic acid). This reaction creates carbon dioxide (and some other things) and breaks down the eggshell in the process. The membrane underneath the shell doesn’t react, so it’s left behind. Once the shell is completely gone, all that’s left is the flexible membrane, giving you a bouncy “rubber” egg!
Learn More: Egg in Vinegar Experiment
Glowing Bouncy Egg

This experiment is just like the egg in vinegar experiment but the addition of fluorescent ink and a black light makes the egg glow! The vinegar dissolves the egg shell leaving a thin membrane. Since membranes let some stuff pass through (like the water in the vinegar and the highlighter fluid) some of the fluorescent molecules travel into the egg. When you shine a black light on the rubbery egg, the fluorescent molecules glow in the dark.
Learn more: Glowing Bouncy Egg
Rock Candy

In this experiment you’ll learn about crystal-growing science while making edible sweet treats. When you combine sugar and boiling water, you end up with a supersaturated solution — that means it has a lot more dissolved sugar particles than normal, room-temperature water would. When the supersaturated solution cools, the water can no longer hold all of the sugar particles, so they start to fall out of the solution. By adding a skewer or string to the solution, the sugar particles have something to attach to. Overtime, the sugar particles combine and crystallize into rock candy!
Learn more: Rock Candy
Celery Experiment

How do plants get water from their roots all the way to their leaves? The answer is tiny tubes inside the stem called the xylem. They draw the water up from the roots like a straw by a process called capillary action. This simple experiment shows capillary action… in action!
Learn more: Celery Experiment
Magic Mug Cake

Normally, a cake would take an hour or more to make in an oven, but with a microwave oven, you can make one in minutes! Microwave ovens use waves of energy called – you guessed it – microwaves to cook food quickly. The microwaves go into the food and make water molecules inside move around really fast. The movement creates heat which cooks the food. Make this delicious chocolate mug cake and experiment by adding other ingredients.
Learn more: Magic Mug Cake
Magnetic Cereal

Magnets are made of a special alloy – or mixture – of three types of metal (neodymium, boron, and iron), which allows them to create a magnetic field. This magnetic field pulls on other magnets and metals, like the iron in your cereal!
Learn more: Magnetic Cereal
Spare Some Spices, Soap and Socks for science
Pepper Swim-Away Experiment

Use this experiment to show your kids the strength of soap! Pepper floats on the surface of water due to surface tension. That means the molecules on the surface of the water hold onto each other so tightly that they create a strong layer that keeps the pepper afloat. But when soap is dropped into the water, it breaks the surface tension and the pepper pieces shoot away!
Learn more: Pepper Swim-Away Experiment
Sensory Lab

What ingredients and herbs does your family use in their everyday cooking? Try mixing and matching some of the spices and scents to discover your favorite combinations and create a sensory lab!
Learn more: Sensory Lab
Unpoppable Bubbles

Make super strong bubbles that won’t want to pop! Bubbles are made up of three layers. The outside and inside walls consist of soap molecules and between them is a really thin layer of water. Bubbles pop on their own when the water between the soapy walls evaporates. Adding soap and corn syrup to water helps make stronger bubbles, because soap and corn syrup molecules can squeeze in between water molecules and help the film of water stretch out without breaking!
Learn more: Unpoppable Bubbles
One-of-a-Kind Bubble Bottle

Want more bubbles? Grab soap, a (clean) sock and a plastic bottle to make a ton of bubbles at once! When you attach a sock to the end of a plastic bottle, it acts like a bubble wand. The bubbles push through the tiny holes in the fabric and connect into a super string of soapy suds!
Learn more: One-of-a-Kind Bubble Bottle
Underwater Fireworks

In this experiment, you use four different liquids with four different densities: oil, water, food coloring, and salt water. The oil sits on top of the water because it’s less dense than water. The water sits on top of the salt water for the same reason. Food coloring is denser than oil and a little bit denser than water, but it isn’t as dense as salt water. When the drops of food coloring hit the dense salt water, they disperse like exploding fireworks!
Learn More: Underwater Fireworks
Got a Bunch of Baking Soda or Borax?
Instant Sensory Snow

Make it snow indoors by mixing together baking soda and shampoo! For extended fun, add food coloring to create vibrantly colored snow, find small toys to create an arctic habitat in the snow, or build mini snowmen with tiny button accessories!
Learn more: Instant Sensory Snow
Bouncy Ball
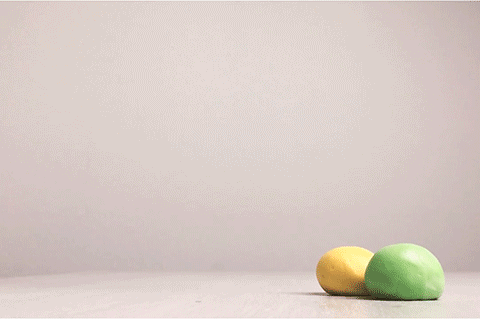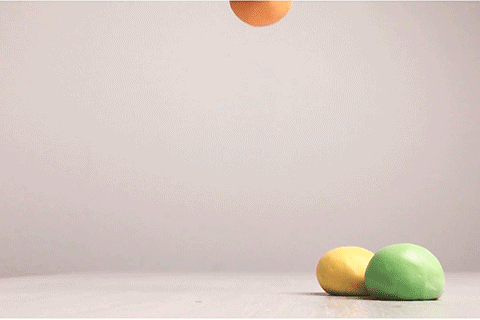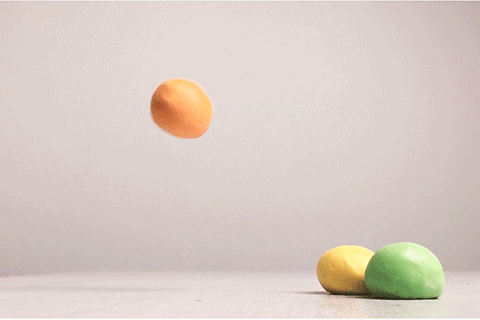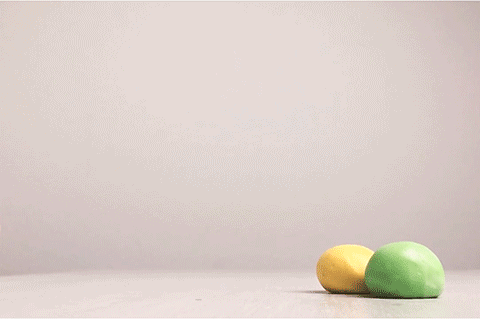
Glue contains some super-long molecules called polymers. In the glue, these polymers are free to float around without getting tangled up with each other. But when you add borax, a chemical reaction takes place. That reaction causes the long polymer molecules to stick to each other. This makes it impossible for the molecules to slide past each other, and that’s what turns the liquid glue into a bouncy ball. This type of reaction is called cross-linking. Real rubber balls also get their bounce from cross-linked polymers!
Learn more: Bouncy Ball
Baking Soda-Powered Boat

Fizz, fizz, zoom! This baking soda experiment boat is easy to build and fun to race. If you’ve ever dropped a fizzy tablet into a cup of water or made a baking soda volcano, you’ve made the same chemical reaction used here. But this time, we’re using that reaction to power a soda bottle boat!
Learn more: Baking Soda-Powered Boat
Magic Inflating Balloon

Use a chemical reaction to inflate a balloon without using your hands! Acids (like vinegar) and bases (like baking soda) do something special when they’re combined. This chemical reaction creates something completely new: carbon dioxide gas! That’s what will bubble up and create all the foamy fizz in your bottle. And once you stretch the balloon over the bottle’s mouth, you create an airtight seal. Then the gas flows up into the balloon, causing the balloon to inflate!
Learn more: Magic Inflating Balloons
Upcycle Used Items & Food Scraps
Eggheads
These little eggheads are an adorable project! Just plant the grass seeds and watch the hair grow. You can even use these eggheads as seed-starter pots because they are biodegradable and full of calcium for your plants!

Learn more: Eggheads
Water Flow Experiment in a Bag

Water likes to stick to surfaces or objects (this is called adhesion), which is why a piece of yarn will emerge wet after being submerged in water. Water molecules also like to stick to each other (this is called cohesion), which is why water poured from a bag will cling to the wet yarn and follow the length of string in a gravity-defying stream.
Learn more: Water Flow Experiment in a Bag
Expanding Star

This growing star trick works thanks to wood’s ability to absorb water. The water soaked into toothpicks and caused the wood to swell, just like a sponge expands when you soak it in water. The expanding wood caused the toothpicks to start to straighten. Because all of the toothpicks push against each other, they opened up to form the star shape you saw.
Learn more: Expanding Star
Sink or Float?

This simple sanity saver is great for entertaining the little ones. Have your child collect some water-safe toys and items around the house to test out which ones will stay afloat.
Learn more: Sink or Float
