Keep your kids’ brains busy and inspire innovation with simple STEAM projects you can do with the materials you have at home!
Just Added!
Homemade Marshmallows
Marshmallows are made by beating together gelatin (or other gelling ingredient) with a hot sugary syrup. When you beat the mixture, you create air bubbles. These bubbles become trapped as the hot liquid cools into a gel creating the fluffy texture. Marshmallows are mostly sugar, but air actually makes up more than half their volume!
Learn more: Homemade Marshmallows
Glow in the Dark Gelatin
Why does this gelatin glow? The active ingredient in tonic water is quinine, a fluorescent substance. Fluorescent substances absorb energy from other light sources and then release this energy as light in its natural state. In this case, the quinine absorbs the energy from the UV light and emits some of that energy back in the form of a visible blue light. This is what gives your gelatin its glow!
Learn more: Glow in the Dark Gelatin
Water Wheel

Create a waterwheel to learn how to convert the energy of falling water into a power source! The water wheel is an ancient device that converts the flow of water into the motion of a wheel. Falling water pushes down on the cups of the water wheel, turning the wheel in one direction. As the cups move around the wheel, they dump out their water, preparing them to be filled back up when they’ve rotated back to the top of the wheel. Water wheels provided mechanical power to communities around the world starting around 400 BCE, helping people pound wheat into flour or saw logs in half. Today, modified water wheels, known as turbines, can be found inside of hydroelectric dams, where they convert the motion of falling water into electricity.
Learn more: Water Wheel
Water Cycle Bags
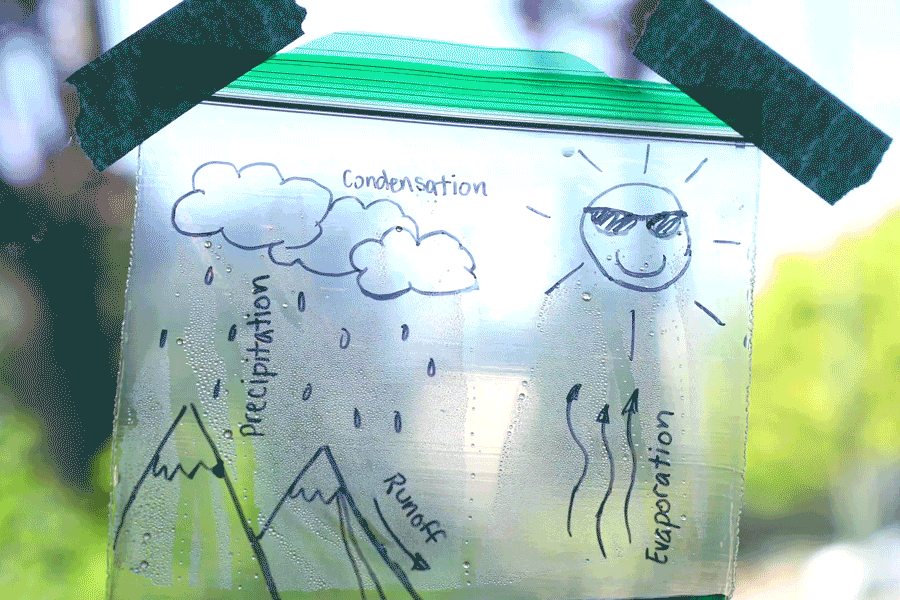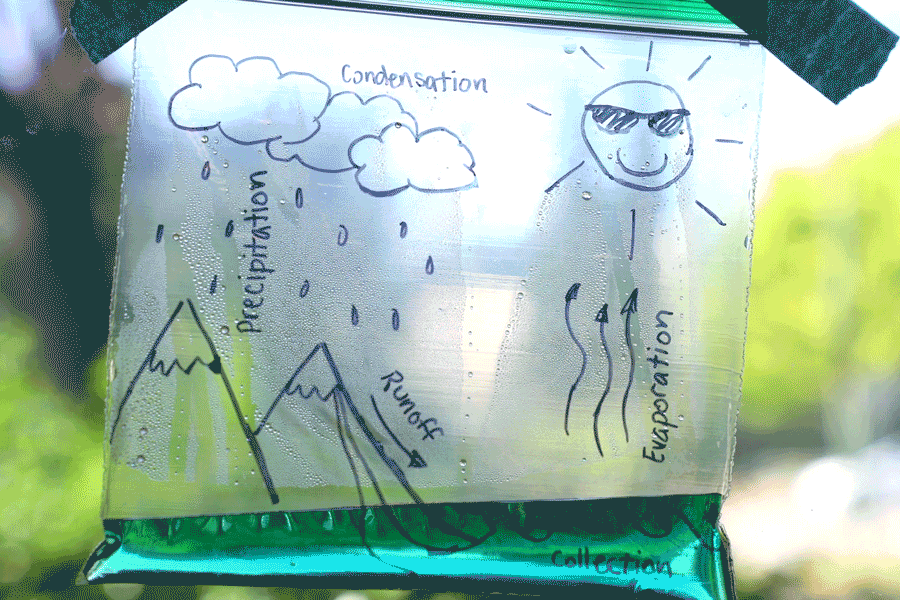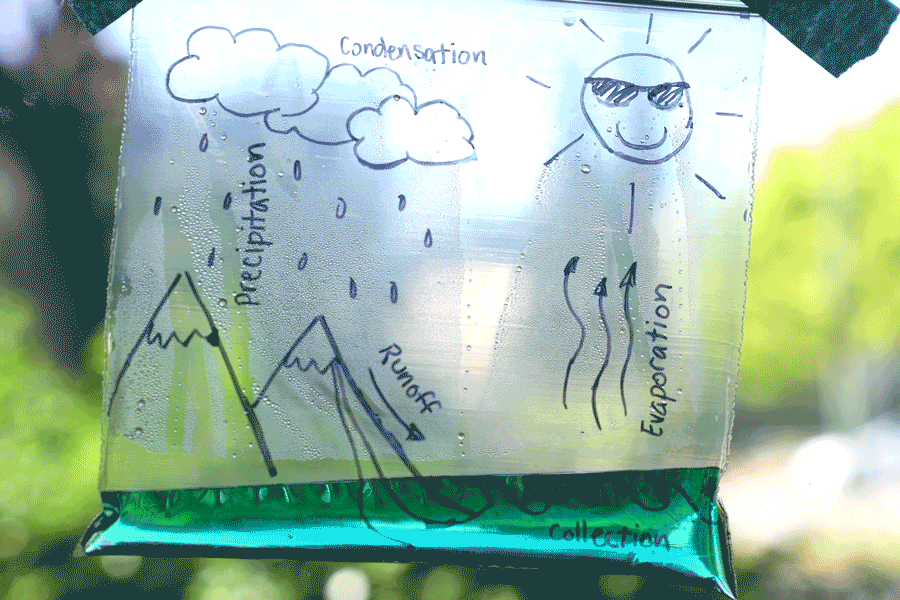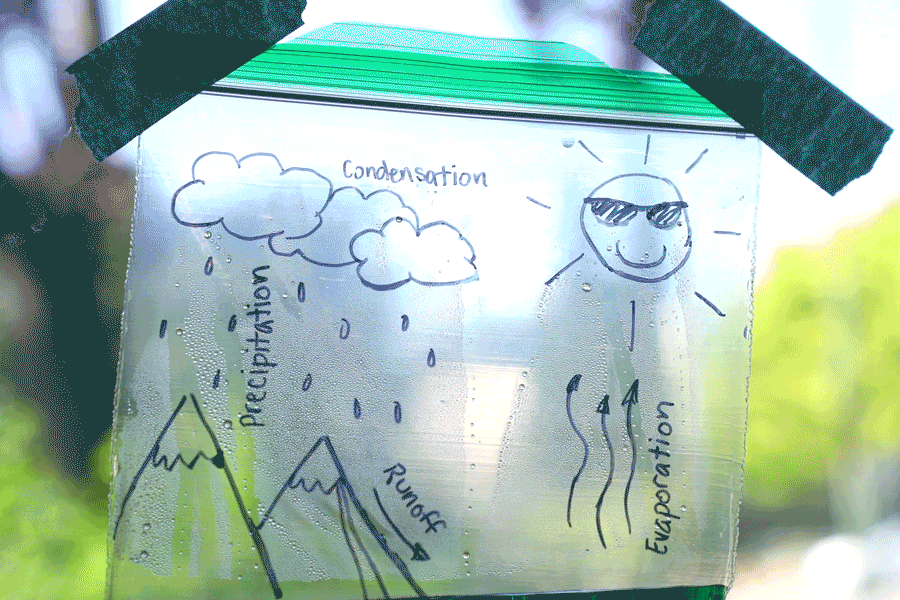
Create a water cycle bag and learn about earth science! Observe how water is influenced by many things, such as energy from the sun, temperature, and the force of gravity. Try creating a second bag and placing it in a different location (perhaps a shady window) and see how the results differ. You can also leave the bag for a few days and check on it at different stages. See how time of day, temperature, and amount of sunlight affects it!
Learn more: Water Cycle Bags
Explore Different Sciences Using Straws
Pumping Heart

Ever wondered how our hearts pump blood? Well, in order to keep your blood flowing in the right direction, your heart has two pretty cool design features: chambers and valves. The chambers fill with blood, then squeeze tight to pump it out. Each chamber also has an exit door called a valve. These keep blood from getting pumped backwards. Build your own model to learn about the right atrium and ventricle!
Learn more: Pumping Heart
Lung Model

Breath in. Breathe out. Do you feel your chest expand and shrink when you breathe? That change in size is how you get air into your lungs! It all has to do with the physics of air pressure, which you’ll investigate in this project. Air always tries to balance out pressure, so it will move from areas of high pressure to areas of low pressure. Your body uses this fact to move air in and out when you breathe! See it in action as you inflate and deflate balloon lungs with this model!
Learn more: Lung Model
Pythagoras Cup

Make your own Pythagoras cup and trick your family with this disappearing water act!
The Pythagoras cup is a fun example of a siphon — a device that uses gravity to drain a container of liquid. As the cup is filled with water, the short end of the straw starts to fill up, reaching the same height as the water in the cup. Once that water level reaches the bend at the top of the straw, some of the water begins to drain down the long end of the straw due to the pull of gravity, so the straw acts like a siphon!
Learn more: Pythagoras Cup
Test Out the Effects of Temperature
Flying Tea Bag

Make a tea bag fly with heat! The flying tea bag experiment is a similar concept to a hot air balloon, but you can do it right at home. This project is simple to create, but impressive to kids and adults alike!
Learn more: Flying Tea Bag
Eggs in a Bottle

With this experiment, you’ll learn how to harness the power of expanding and contracting gasses to suck an egg into a bottle in which it would never normally fit. As the flame burns inside the bottle, it heats up the air around it, causing it to expand. If you saw your egg vibrating slightly, this was because air was escaping from the bottle. When the flame goes out, the air in the bottle cools and shrinks. This is what sucks your egg into the bottle!
Learn more: Eggs in a Bottle
Hot Hand Ice Warmers

All you need is baking soda and vinegar to create a little chemistry experiment that comes handy on cold days!
Learn more: Hot Hand Ice Warmers
Bottle Thermometer

The thermometer you’re going to make uses alcohol to read temperature. Alcohol changes its size when its temperature changes, even though you might not see it happen when the alcohol just sits in the bottle. The trick for making it noticeable is to put the alcohol in a thin tube, like a straw. With less space inside the straw, even a small change in the alcohol’s size makes a big difference to how high or low the top of the liquid is. The alcohol always goes to the same level for the same temperature, so you can tell how hot or cold something is by comparing to different measurements of the alcohol level.
Learn more: Bottle Thermometer
Cook with Chemistry & Experiment with Food
Fluorescent Frosting

Tonic water makes your cupcakes glow because of a chemical in it called quinine. People originally put quinine in drinks because it can help to prevent malaria. Quinine also turns out to be fluorescent, which means that it glows when it’s hit by ultraviolet light. With just a bit of tonic water, your cupcakes will really stand out under a blacklight!
Learn more: Fluorescent Frosting
Fluorescent Jello

Double up your experiment and test out the same recipe with jello!
Learn more: Fluorescent Jello
Fizzy Candy

This homemade version of the classic pop rocks will get you fizzy with baking soda and citric acid! Personalize this candy with your own flavor and experience this chemical reaction in your mouth! This recipe keeps the citric acid and baking soda separate until you eat it. When these two ingredients combine with the saliva in your mouth, it creates carbon dioxide gas. It doesn’t “pop” like the fizzy candy sold in stores, but you are still experiencing a chemical reaction in your mouth!
Learn more: Fizzy Candy
Maple Syrup Crystals

Test out the effects of temperature with this sweet science experiment! When you pour hot maple syrup onto a cold pan, sugar molecules slow down, combine, and then harden into solid crystals. The best part is, you can eat the crystals once you’re done with the experiment!
Learn more: Maple Syrup Crystals
Yummy Polymer Gummies

Combine a lesson on polymers with a delicious snack food! You can picture the molecules that make up gelatin as long, twisty chains called polymers. When you heated up the gelatin and water mixture, the heat caused those chains to break apart into smaller pieces. When the mixture cools, the broken-up chains start to stick to each other again. But they do this in a way that creates tiny pockets in between them. Tiny drops of water get trapped in those pockets, which creates a yummy gummy texture!
Learn more: Yummy Polymer Gummies
Cabbage Chemistry

Color your world with cabbage and learn about chemistry! The terms acid and base describe chemical properties of many things we use everyday. Sometimes, you can tell if something is an acid or base by the way it tastes. Instead of a taste test, chemists use a pH scale to measure the strength of acids and bases. In this project, you’ll test different substances in purple cabbage juice and compare the results to a printable pH scale.
Learn more: Cabbage Chemistry
Turn Milk into Cheese

Milk is made up of proteins, sugars, fat, minerals, vitamins, and enzymes. When you add an acid like lemon juice to warm milk, it causes molecules of one of the proteins in milk to bond to one another. That forms a solid lump of protein which is also known as a cheese curd and the leftover liquid is called whey. You may remember hearing about curds and whey from the old nursery rhyme about Little Miss Muffet!
Learn more: Turn Milk into Cheese
Baked Custard

Learn about the chemistry of eggs with this sweet experiment. Raw egg proteins are shaped like tightly packed balls, kind of like a long string that’s been crumpled up. But when you heat them (in a custard mixture) the egg proteins unfurl. As more heat is added these long protein strands unfold and begin to grab on to each other. Eventually, a mesh of protein strands is formed. The fat, sugar, and liquids are trapped in the pockets in between the strands, forming the lovely delicious texture of a perfectly baked custard.
Learn more: Baked Custard
Dancing Salt

Discover how music creates vibrations you can see using salt and a portable speaker! Speakers produce sound by creating vibrations in the air. Normally, we only hear these vibrations, and we can’t easily see them. Plastic wrap, though, is lightweight and thin enough to vibrate in response to the sounds coming from the speakers. These vibrations move through the plastic wrap unevenly, pushing and shoving the salt around in interesting patterns as if it were dancing!
Learn more: Dancing Salt
Salty Cave Crystals

In nature, crystals often form when hot substances — like magma — start to cool and harden. During this process, known as crystallization, atoms in the substance begin to stick together in repeating patterns. The end result is the creation of unique geometric crystals! You can make crystals at home through a similar process using hot water and epsom salt. Mixing salt and hot water creates a supersaturated solution — that means it has a lot more dissolved salt particles than normal, room-temperature water would. When the supersaturated solution cools, the water can no longer hold all of the salt particles, so they start to fall out of the solution. Overtime, the salt particles combine on the string and crystallize!
Learn more: Salty Cave Crystals
Potato Chip Patina Experiment

Have you ever wondered why the Statue of Liberty is green? It’s because of a process called oxidation – a natural weathering process that occurs when air and water react with copper! Try this experiment to recreate the oxidation process artificially!
Learn more: Potato Chip Patina Experiment
Magic Mug Cake

Normally, a cake would take an hour or more to make in an oven, but with a microwave oven, you can make one in minutes! Microwave ovens use waves of energy called – you guessed it – microwaves to cook food quickly. The microwaves go into the food and make water molecules inside move around really fast. The movement creates heat which cooks the food. Make this delicious chocolate mug cake and experiment by adding other ingredients.
Learn more: Magic Mug Cake
Get Creative with Food Coloring
Bubble Lamp in a Bag

Oil and water famously don’t mix well. No matter how much you stir them together, they’ll always separate as oil rises to the top. But oil and water don’t avoid mixing because they don’t like each other; it’s because of their chemistry! In this project, you’ll learn more about the chemistry of oil and water and create a bubbly chemical reaction — both essential to making your Bubble Lamp in a Bag super groovy and fun.
Learn more: Bubble Lamp in a Bag
Magic Water Barrier

Learn about how the density of water changes with different temperatures in this colorful science project. This experiment explores the difference in density between hot and cold water. When water is heated up, its water molecules move more quickly, expanding the space between individual molecules. This increases the water’s overall volume and decreases its density.
Learn more: Magic Water Barrier
Underwater Fireworks

In this experiment, you use four different liquids with four different densities: oil, water, food coloring, and salt water. The oil sits on top of the water because it’s less dense than water. The water sits on top of the salt water for the same reason. Food coloring is denser than oil and a little bit denser than water, but it isn’t as dense as salt water. When the drops of food coloring hit the dense salt water, they disperse like exploding fireworks!
Learn More: Underwater Fireworks
Upcycle Materials Around Your House
Magic Cloud
Whether they’re bringing down rain or snow, making a beautiful sunset, or letting our minds run wild with imaginary shapes – clouds are pretty awesome. Did you know that you can create your own cloud in a bottle with just a few easy steps? Follow along with the instructions or watch our video tutorial!
Learn more: Magic Cloud
DIY Phone Speaker

Make a rockin’ customizable speaker for you and your friends to enjoy with just a few household items! Understand how sound vibrations can travel through different mediums and how the shape of the mediums can cause the sounds to be amplified!
Learn more: DIY Phone Speaker
Craft Stick Reaction

Chain reactions are amazing displays of energy. When everything is set up right, one little tap can cause a cascade of action, like a single domino knocking over a chain of thousands. Try this experiment to make a huge chain reaction out of just a few craft sticks!
Learn more: Craft Stick Reaction
